Answer:
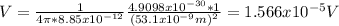
And we can express the last expression like this:
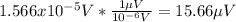
Step-by-step explanation:
For this case we know the dipole moment given by 1.47 D. We know also that
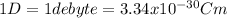
And we need to calculate the electric potential due to an ammonia molecule at a point 53.1 nm away along the axis of the dipole
We can convert the dipole moment like this:
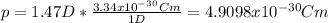
And the potentital at the desired point can be calculated with the following formula:
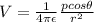
Where
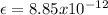 is a constant,
is a constant,
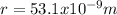 , and since we are assuming V=0 we can conclude that
, and since we are assuming V=0 we can conclude that
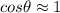
If we replace the info given we got:
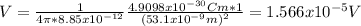
And we can express the last expression like this:
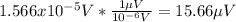
And this would be our final answer for this case.