Answer:
t = 2,57 days
Step-by-step explanation:
A reaction follows the first-order kinetics when attend the formula:
![ln([A]_t)/([A]_0) = -kt](https://img.qammunity.org/2021/formulas/chemistry/college/2ugl4j6gys6qes7j58w6c6l35dcxvo2ohx.png)
Where [A]₀ is the initial concentration of the toxin (0,63mg/L) and [A]t is the half of the initial concentration (0,315mg/L). k is 0,27 day⁻¹ and t is the time the descomposition takes. Replacing:
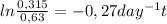
t = 2,57 days
I hope it helps!