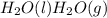Step-by-step explanation:
In a chemical equilibrium, there are different reactants and products are involved. For example, reaction equation for self-ionization is as follows.
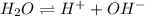
This reaction is a chemical equilibrium. Also, nitrogen dioxide when present in equilibrium with nitrogen tetraoxide represents chemical equilibrium.
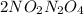
On the other hand, in a physical equilibrium same substance is present in different physical states. For example, when ice is present in equilibrium with water then it shows physical equilibrium.
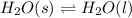
And, liquid water present in equilibrium with water vapor also represents physical equilibrium.