Complete Question
The complete question is shown on the first uploaded image
Answer:
Answer: 760 uM
Step-by-step explanation:
the addition of solvent to a solution in a such away that the volume of the solution increases and the concentration decreases is a process know as dilution
The concentration and the volume of the dilute and concentrated solution are given below as follows
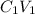
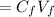
Concentrated solution Dilute solution
Given that M1 = 97.0uM
V1 = 47.0 mL
V2 = 6.0 mL
M2 = ?
Therefore M1V1 = M2V2
M2 = M1V1/V2
M2 = (97*47)/6
M2 = 760 uM
Answer: 760 uM