Answer:
The percent yield is 90%
Step-by-step explanation:
First, we write the combustion reaction.
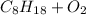 →
→
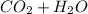
Then we balanced the equation.
2
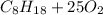 →
→
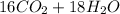
Now, we calculate the moles of octane.
n(octane)=
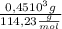
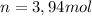
Now we find the theoretical moles of C02, taking into account stoichiometric coefficients
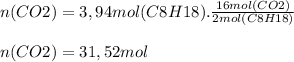
Finally, we calculate the mass of CO2 and proceed to calculate the percent yield
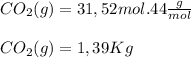
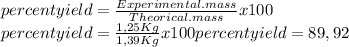
the percent yield is 89,92%≈90%