Answer: The theoretical yield and percent yield of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) is 3.93 g and 30.53 % respectively
is 3.93 g and 30.53 % respectively
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
Given mass of
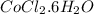 = 4.00 g
= 4.00 g
Molar mass of
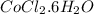 = 238 g/mol
= 238 g/mol
Putting values in equation 1, we get:
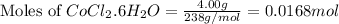
The chemical equation for the reaction of
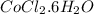 to form
to form
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) follows:
follows:
![CoCl_2.6H_2O+4NH_3\rightarrow [Co(NH_3)_4(H_2O)_2]Cl_2+4H_2O](https://img.qammunity.org/2021/formulas/chemistry/college/e9tzeo6h3aymznf5f4dj2noifw9g25x1vg.png)
By Stoichiometry of the reaction:
1 mole of
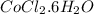 produces 1 mole of
produces 1 mole of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png)
So, 0.0168 moles of
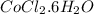 will produce =
will produce =
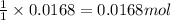 of
of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png)
Now, calculating the mass of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) from equation 1, we get:
from equation 1, we get:
Molar mass of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) = 234 g/mol
= 234 g/mol
Moles of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) = 0.0168 moles
= 0.0168 moles
Putting values in equation 1, we get:
![0.0168mol=\frac{\text{Mass of }[Co(NH_3)_4(H_2O)_2]Cl_2}{234g/mol}\\\\\text{Mass of }[Co(NH_3)_4(H_2O)_2]Cl_2=(0.0168mol* 234g/mol)=3.93g](https://img.qammunity.org/2021/formulas/chemistry/college/9xbspazfttk2cb73nyzijy89hgmfwayh4q.png)
To calculate the percentage yield of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) , we use the equation:
, we use the equation:
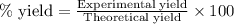
Experimental yield of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) = 1.20 g
= 1.20 g
Theoretical yield of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) = 3.93 g
= 3.93 g
Putting values in above equation, we get:
![\%\text{ yield of }[Co(NH_3)_4(H_2O)_2]Cl_2=(1.20g)/(3.93g)* 100\\\\\% \text{yield of }[Co(NH_3)_4(H_2O)_2]Cl_2=30.53\%](https://img.qammunity.org/2021/formulas/chemistry/college/i5qvkg21mgmzzjr7oa009e5t5swc0y9b2d.png)
Hence, the theoretical yield and percent yield of
![[Co(NH_3)_4(H_2O)_2]Cl_2](https://img.qammunity.org/2021/formulas/chemistry/college/lhlpxkca2pihlz9z4y630apsp5uyovybzm.png) is 3.93 g and 30.53 % respectively
is 3.93 g and 30.53 % respectively