Answer:
Keq=0.866
Step-by-step explanation:
Hello,
In this case, the undergone chemical reaction is:
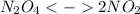
In such a way, since 0.0055 mol of N₂O₄ remains in the flask, one infers that the reacted amount (
 ) was:
) was:
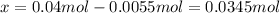
In addition, the produced amount of NO₂ is:
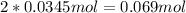
Finally, considering the flask's volume, the equilibrium constant is then computed as follows:
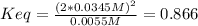
Best regards.