Answer:
The last statement describes the correct inozation of water:
Pure water at 25ºC:
- self-ionizes to form an equilibrium system in which:
![[H_3O^+]=[OH^-]=10^(-7)moles/liter](https://img.qammunity.org/2021/formulas/chemistry/middle-school/l5alzpvr8zb5m0ut76jcef8zqwq0uie04d.png)
Step-by-step explanation:
It has been proven that pure water slightly conducts electricity. This fact, explained by the Arrhenius model of acids, would mean that pure water contains ions.
Since such ions are spontaneoulsy produced by the water molecules, this phenomenum is called self-ionization of water.
The equilibrium equation that represents the self-ionization of water is:
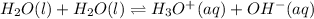
The expression for the equilibrium constant is:
![Keq=[H_3O^+(aq)][OH^-(aq)]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/542bu0t73mg9qep63grzj2vp6zh0r2tpx7.png)
As per the stoichiometry:
![[H_3O^+(aq)]=[OH^-(aq)]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/utjo1uz7cjj28gh7809izn22tvcju5xdq0.png)
The equilibrium constant for the self-ionization of water has been determined at several temperatures. At 25ºC it is equal to 1.0×10⁻¹⁴.
Then by solving the equation you can find the concentrations of the ions:
![[H_3O^+(aq)]\cdot [OH^-(aq)]=10^(-14)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/14ekqdnoolyj2q7lqffyqv9fa1l4dypchn.png)
![[H_3O^+(aq)]=[OH^-(aq)]=x\\\\x^2=10^(-14)\\\\x=\sqrt{10^(-14)}\\\\x=10^(-7)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/ic2695kdg7eqm046139laqkuiluf5b8gxi.png)
![[H_3O^+(aq)]=[OH^-(aq)]=10^(-7)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/kaqja0r1o4v3h5lrd40qvvrfgiq6spbuzc.png)
Hence, we have proved that pure water self-ionizes to form an equilibrium system in which:
![[H_3O^+]=[OH^-]=10^(-7)moles/liter](https://img.qammunity.org/2021/formulas/chemistry/middle-school/l5alzpvr8zb5m0ut76jcef8zqwq0uie04d.png)