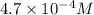Answer: The concentration of hydrogen ions is
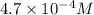
Step-by-step explanation:
We are given:
Concentration of acid = 0.22 M
The chemical equation for the dissociation of monoprotic weak acid follows:
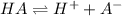
Initial: 0.22
At eqllm: 0.22-x x x
The expression of
 for the above equation follows:
for the above equation follows:
![K_a=([H^+][A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/cd33ttbdpkk5h562zi8dnbyn0akiej88j5.png)
We are given:
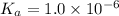
Putting values in above equation, we get:
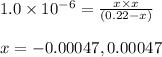
Neglecting the negative value of 'x', because concentration cannot be negative
So, equilibrium concentration of hydrogen ions = x = 0.00047 M =
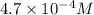
Hence, the concentration of hydrogen ions is