Answer:
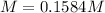
Step-by-step explanation:
Hello,
In this case, a mixing process is carried out, so the molarity should be modified by considering the new volume of the solution after mingling, in such a way, the resulting moles are:
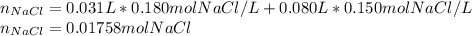
Afterwards, the total new volume of the solution is:
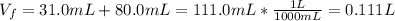
Finally, the new molality is:
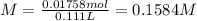
Best regards.