The question is incomplete, here is the complete question:
A 50 mL solution is initially 1.52% MgCl₂ by mass and has a density of 1.05 g/mL
What is the freezing point of the solution after you add an additional 1.37 g MgCl₂? (Use i = 2.5 for MgCl₂).
Answer: The freezing point of solution is -0.808°C
Step-by-step explanation:
To calculate the mass of solution, we use the equation:
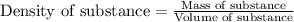
Density of solution = 1.05 g/mL
Volume of solution = 50 mL
Putting values in above equation, we get:
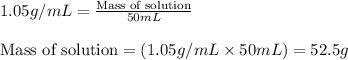
We are given:
Percentage of magnesium chloride in the solution = 1.52 %
Mass of magnesium chloride in the solution = 1.52 % of 52.5 g =
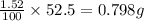
The equation used to calculate depression in freezing point follows:
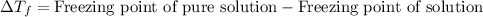
To calculate the depression in freezing point, we use the equation:
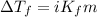
Or,
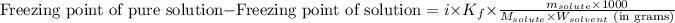
where,
Freezing point of pure solution (water) = 0°C
i = Vant hoff factor = 2.5
 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m
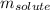 = Given mass of solute (magnesium chloride) = [0.798 + 1.34] g = 2.138 g
= Given mass of solute (magnesium chloride) = [0.798 + 1.34] g = 2.138 g
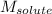 = Molar mass of solute (magnesium chloride) = 95.2 g/mol
= Molar mass of solute (magnesium chloride) = 95.2 g/mol
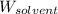 = Mass of solvent (water) = [52.5 - 0.798] g = 51.702 g
= Mass of solvent (water) = [52.5 - 0.798] g = 51.702 g
Putting values in above equation, we get:
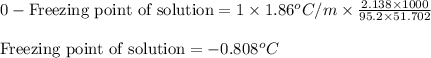
Hence, the freezing point of solution is -0.808°C