Answer:
option a
Step-by-step explanation:
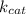 is turnover number. The turnover number is defined as the number of chemical conversions by single enzymatic site per second.
is turnover number. The turnover number is defined as the number of chemical conversions by single enzymatic site per second.
K_m is Michaelis-Menten constant used in the study of kinetics of enzyme catalyzed reaction.
The relationship between Michaelis-Menten constant and turnover number is as follows:
/(K_m+[S])](https://img.qammunity.org/2021/formulas/chemistry/high-school/f8vksp7nmk4xwljulguwcybr8vr7dxgp5j.png)
Here, [S] is substrate concentration, E0 is the initial enzyme concentration and v is the rate of enzyme catalyzed reaction.
Turn over no. (
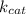 ) is present in nominator while K_m is present in denominator. Therefore for lowest catalytic efficiency, turnover (
) is present in nominator while K_m is present in denominator. Therefore for lowest catalytic efficiency, turnover (
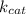 ) should be low and Michaelis-Menten constant (K_m) should be high.
) should be low and Michaelis-Menten constant (K_m) should be high.
Therefore, among, given option a is correct.