Answer:
1) The overall chemical equation will be :
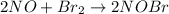
2) The species which acting as a intermediate is
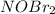 .
.
3) The rate law for the overall reaction :
![R=K[NO]^2[Br]](https://img.qammunity.org/2021/formulas/chemistry/college/rym1mmlur2ecstzghfi950sgaqod7up502.png)
Step-by-step explanation:
1) Step 1 : Fast:
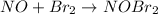 ..[1]
..[1]
Step 2: Slow:
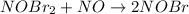 ...[2]
...[2]
The overall chemical equation will be :
[1] + [2]
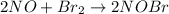
2) Intermediate are those chemical species are which formed in between the process of chemical reaction , but do not appears in overall reaction. They act as a product and reactant in the process where chemical reaction taking place in more than one step.
The species which acting as a intermediate is
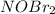 .
.
3) Rate law for the overall reaction that is consistent with this mechanism:
When the reaction is taking place in more than one step than the rate of the reaction is determined by the step in which reaction progress is slow.
So, for the given mechanism the rate of the overall chemical creation will be determined by step 2:
Step 2: Slow:
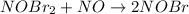 ...[2]
...[2]
Rate of the reaction :
![R=k[NOBr_2][NO]](https://img.qammunity.org/2021/formulas/chemistry/college/mfumhcx2qt6m6ab226m0kboepg6msqyp8k.png) ..[3]
..[3]
From step 1 :
![K_c=([NOBr_2])/([NO][Br_2])](https://img.qammunity.org/2021/formulas/chemistry/college/twuox869m84fisjgkz923a5o350rcph8na.png)
![[NOBr_2]=K_c* [NO][Br_2]](https://img.qammunity.org/2021/formulas/chemistry/college/6pljwgy9jmv6xl6ctixa42guii8i4x9s8s.png)
Putting value of
![[NOBr_2]](https://img.qammunity.org/2021/formulas/chemistry/college/5tyw66aeyazqsyjcish9momnslew498eak.png) rate expression [3]:
rate expression [3]:
![R=k* k_c[NO][NO][NO]=K[NO]^2[Br]](https://img.qammunity.org/2021/formulas/chemistry/college/l10p626j7hjg6hcscxwskl4neqaevewt70.png)
Where :
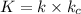
The rate law for the overall reaction :
![R=K[NO]^2[Br]](https://img.qammunity.org/2021/formulas/chemistry/college/rym1mmlur2ecstzghfi950sgaqod7up502.png)