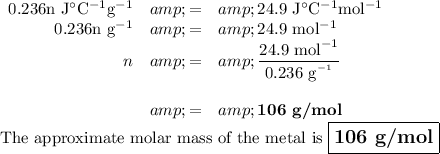Answer:
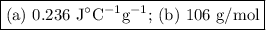
Step-by-step explanation:
a) Specific heat capacity of the metal
The guiding principle in calorimetry is the Law of Conservation of Energy: the sum of all the energy transfers must add up to zero. That is,
q₁ + q₂ + … = 0
The formula for the heat q gained or lost by a substance is
q = mCΔT
where
m = the mass of the substance.
C = its specific heat capacity.
ΔT = the change in temperature.
In this problem, there are two heat transfers: the heat lost by the metal and the heat gained by the water.
m₁C₁ΔT₁ + m₂C₂ΔT₂ = 0
Data:
m₁ = 58.215 g; T₁ = 99.0 °C; T₂ = 27.2 °C
m₂ = 41.202 g; T₁ = 21.5 °C; T₂ = 27.2 °C
Calculations:
ΔT₁ = 27.2 - 99.0 = -71.8 °C
ΔT₂ = 27.2 - 21.5 = 5.7 °C
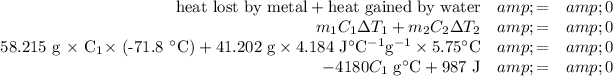
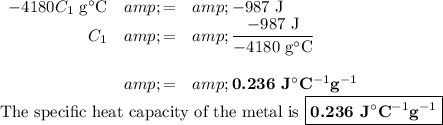
b) Approximate molar mass of the metal
We could look up the metal that has the calculated specific heat capacity, but that would give the exact molar mass.
They asked for the approximate value, do they are probably expecting you to do something else.
Per the Law of Dulong and Petit, the molar specific heat capacity of a metal should be about 24.9 J·°C⁻¹mol⁻¹