Answer:
4.25% is the final concentration of phosphoric acid.
Step-by-step explanation:
Initial concentration of phosphoric acid =
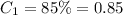
Initial volume of phosphoric acid =
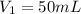
Final concentration of phosphoric acid =
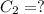
Final volume of phosphoric acid =
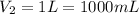
( 1L = 1000 mL)
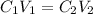
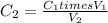
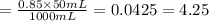
4.25% is the final concentration of phosphoric acid.