Answer : The molar enthalpy of reaction is, 33.3 KJ/mole
Explanation :
First we have to calculate the mass of water.
As we know that the density of water is 1 g/ml. So, the mass of water will be:
The volume of water =
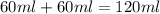
Now we have to calculate the heat absorbed during the reaction.
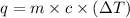
where,
q = heat absorbed = ?
 = specific heat of water =
= specific heat of water =
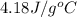
m = mass of water = 120 g
 = change in temperature =
= change in temperature =
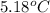
Now put all the given values in the above formula, we get:
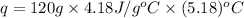
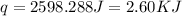
Now we have to calculate the molar enthalpy of reaction.
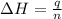
where,
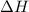 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?
q = heat released = 2.60 KJ
n = number of moles =
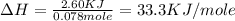
Therefore, the molar enthalpy of reaction is, 33.3 KJ/mole