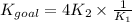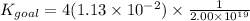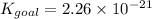Answer:
Part A
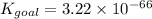
Part B
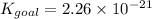
Step-by-step explanation:
Part A:
The given equation is not balanced. So, initially let's balance the equation by taking 24 moles of each of the reagent and NO.
24N₂ + 24H₂O ⇌ 24NO + 12N₂H₄
Now, simplify the equation by dividing both sides by 12. The final balanced equation is the following
2N₂ + 2H₂O ⇌ 2NO + N₂H₄
The above-balanced equation can be solved algebraically to obtain the required Kgoal value.
Adding given equations 1, 2 and 3 we obtain the required equation.
When the equations are added, the equilibrium constants of each equation are multiplied. Mathematically it can be represented as,
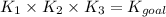
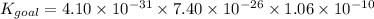
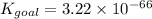
Part B:
The required equation is balanced, Now
Let.
P₄(s)+6Cl₂(g) ⇌ 4PCl₃(g), K₁=2.00×10¹⁹ ------------------------------------ (a)
PCl₅(g) ⇌ PCl₃(g)+Cl₂(g), K₂=1.13×10⁻² --------------------------------------- (b)
By multiplying equation 2 by 4 and subtracting equation 1 from it, we get
4PCl₅(g) ⇌ P₄(s)+10Cl₂(g)
The Kgoal for the above equation is the product of four times K₂ and inverse K₁ according to the applied operation. Mathematically,