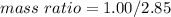Answer:


Step-by-step explanation:
1.
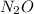
Mass ratio: 1.00 : 0.57 (given)
Represent the atomic mass of N by x and the atomic mass of O by y, then:
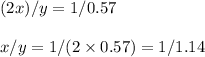
2.
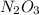
Using x and y for the corresponding atomic masses, the mass ratio of
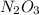 is:
is:
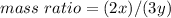
Use x/y = 1/1.14
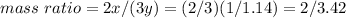
Divide both numerator and denominator by 2:
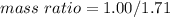

3.
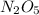
Using x and y for the corresponding atomic masses, the mass ratio of
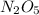 is:
is:
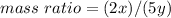
Use x/y = 1/1.14
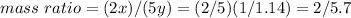
Divide both numerator and denominator by 2: