Answer:
The cuvette was blank with the solution so that the spectrometer will only read the solute absorbance. This also ensures that the spectrometer will ignore other absorbance fluctuations that normally occur due to the chemical make-up of water. The spectrometer only considered the absorbance of
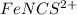 as indicated on the spectrum. The reaction between the
as indicated on the spectrum. The reaction between the
 and the
and the
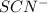 are both clear liquids that form the orange liquid product
are both clear liquids that form the orange liquid product
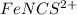 which creates the absorbance spectrum. Because the color of the solution is orange, it reflects this and similar colors while absorbing blueish hues. We can find the absorption of only the
which creates the absorbance spectrum. Because the color of the solution is orange, it reflects this and similar colors while absorbing blueish hues. We can find the absorption of only the
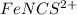 by pre-rinsing the cuvette with each solution we intend to measure before placing it in the spectrometer. Also, wipe each cuvette with a kimwipe to remove all fingerprints that could effect the data collection.
by pre-rinsing the cuvette with each solution we intend to measure before placing it in the spectrometer. Also, wipe each cuvette with a kimwipe to remove all fingerprints that could effect the data collection.
Step-by-step explanation:
The cuvette was blank with the solution so that the spectrometer will only read the solute absorbance. This also ensures that the spectrometer will ignore other absorbance fluctuations that normally occur due to the chemical make-up of water. The spectrometer only considered the absorbance of
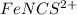 as indicated on the spectrum.
as indicated on the spectrum.