Answer:
0.2212 J/g.k
Step-by-step explanation:
The law of conservation of Energy states that the sum of heat given out by the mineral in the system and the heat absorbed by the water and calorimeter are equal to zero.
This implies that:
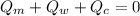
We can as well say that:
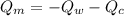 ----------------equation (1)
----------------equation (1)
Also, heat absorbed by the calorimeter which is denoted by;
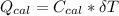 ---------------- equation (2)
---------------- equation (2)
where;
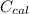 = heat capacity of the calorimeter
= heat capacity of the calorimeter
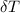 = change in temperature
= change in temperature
However, the heat released by the mineral and the one absorbed by the water in the system is given as:
Q = mcΔT ------------------ equation (3)
where;
m = mass
c = specific heat capacity
ΔT = change in temperature
If we substitute the two prior equation (i.e equation 2 and 3) into equation 1: we have;
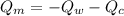
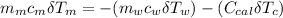
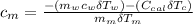 ---------------- equation (4)
---------------- equation (4)
Now , let's state our given parameters;
Given that:
mass of water (
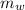 ) = 72g
) = 72g
The specific Heat capacity of water (
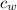 ) = 4.18 J/g.k
) = 4.18 J/g.k
change in temperature (
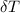 ) =25.6 °C - 20°C
) =25.6 °C - 20°C
change in temperature (
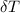 ) = 5.6 °C
) = 5.6 °C
(since we are asked to leave our answer in J/g°C)
Heat capacity of calorimeter
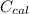 = 14.7 J/k
= 14.7 J/k
change in temperature (
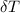 ) = 5.6 °C
) = 5.6 °C
mass of the mineral = 116 g
change in temperature of the mineral (
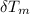 ) = 25.6 °C - 94.5 °C
) = 25.6 °C - 94.5 °C
change in temperature of the mineral (
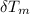 ) = -68.9 °C
) = -68.9 °C
specific heat capacity of the mineral
∴ substituting all our values into equation (4); we have:
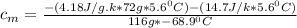
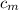 = 0.221172 J/g°C
= 0.221172 J/g°C
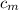 = 0.2212 J/g°C
= 0.2212 J/g°C