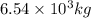Answer : The mass of sodium carbonate added to neutralize must be,
Explanation :
First we have to calculate the moles of
 .
.
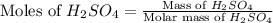
Given:
Molar mass of
 = 98 g/mole
= 98 g/mole
Mass of
 =
=
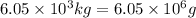
Conversion used : (1 kg = 1000 g)
Now put all the given values in the above expression, we get:
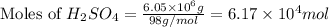
The moles of
 is,
is,
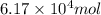
Now we have to calculate the moles of
The balanced neutralization reaction is:
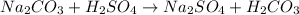
From the balanced chemical reaction we conclude that,
As, 1 mole of
 neutralizes 1 mole of
neutralizes 1 mole of
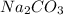
So,
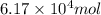 of
of
 neutralizes
neutralizes
Now we have to calculate the mass of
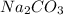
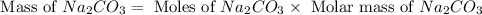
Molar mass of
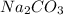 = 106 g/mole
= 106 g/mole
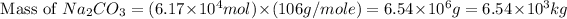
Thus, the mass of sodium carbonate added to neutralize must be,