Answer : The concentration of
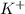 ion in 0.15 M
ion in 0.15 M
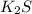 is, 0.3 M
is, 0.3 M
Explanation :
The given compound is,
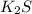
When
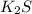 dissociates then it gives potassium ion and sulfide ion.
dissociates then it gives potassium ion and sulfide ion.
The balanced dissociation reaction is:
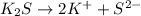
By the stoichiometry we can say that, 1 mole of
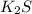 dissociates to give 2 moles of
dissociates to give 2 moles of
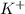 ion and 1 mole of
ion and 1 mole of
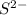 ion.
ion.
Or, in terms of concentration we can say that:
0.15 M of
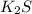 dissociates to give
dissociates to give
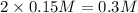 of
of
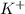 ion and 0.15 M of
ion and 0.15 M of
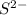 ion.
ion.
Thus, the concentration of
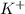 ion in 0.15 M
ion in 0.15 M
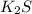 is, 0.3 M
is, 0.3 M