Answer:
Volume of ammonia formed is 7.56 L
Step-by-step explanation:
Volume of 1 mol of gas is 24 L.
No. of mol in 3.78 L of nitrogen =
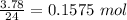
No. of mol in 3.78 L of hydrogen =
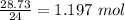
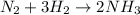
I mol of nitrogen required 3 moles of hhydrogen.
So, 0.1575 mol of nitrogen will require 0.4725 moles of hydrogen.
As moles of hydrogen is 1.197, therefore, nitrogen will be the limiting reagent.
So,
1 mol of nitrogen forms 2 moles of ammonia
0.1575 mol of nitrogen will form 0.1575 mol × 2 = 0.315 mol of ammonia
Volume of ammonia = 0.315 × 24
= 7.56 L
Volume of ammonia formed is 7.56 L