Answer:
75.5g
Step-by-step explanation:
From the ionic equation, we can write
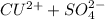
next we find the number of charge
Note Q=it
for i=8.5A, t=3.75 to secs 3.75*60*60=13500secs
hence
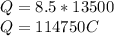
Since one faraday represent one mole of electron which equal 96500C
Hence the number of mole produced by 114750C is
114750/96500=1.2mol
The mass of copper produced is
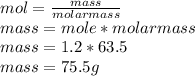
Hence the amount of copper produced is 75.5g