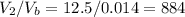Answer:
884 balloons
Step-by-step explanation:
Assume ideal gas, since temperature is constant, then the product of pressure and volume is constant.
So if pressures reduces from 100 to 1.2, the new volume would be
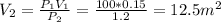
The spherical volume of each of the balloon of 30cm diameter (15 cm or 0.15 m in radius) is
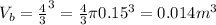
The number of balloons that 12.5 m3 can fill in is