Answer:
the flow rate for steam from the boiler plant =
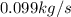
the flow rate from the city water = 0.901 kg/s
the rate of entropy production in the mixing tank = 0.2044 kJ/k
Step-by-step explanation:
In a well-insulated mixing tank where:
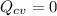 &
&
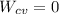
The mass flow rates can be calculated using the formula:
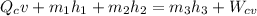 ------ equation (1)
------ equation (1)
so;
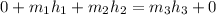 ------- equation (2)
------- equation (2)
Given that:
From the steam in the boiler plant;
The temperature (T₁) = 200°C
Pressure (P₁) = 10 bar
The following data from compressed water and super-heated steam tables were also obtained at: T₁ = 200°C
h₁ = 2828.27 kJ/kg
s₁ = 6.95 kJ/kg K
m₁ (flow rate for steam in the boiler plant) = ????
Also, for city water
The temperature (T₂) = 20°C
Pressure (P₂) = 1 bar
Data obtained from compressed water and super-heated steam tables are as follows:
h₂ = 84.01 kJ/kg
s₂ = 0.2965 kJ/kg K
m₂ (flow rate for city water) = ???
For stream of hot wat at 85 deg C
Temperature (T₃) = 85°C
h₃(
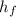 ) = 355.95 kJ/kg
) = 355.95 kJ/kg
s₃(
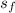 ) = 1.1344 kJ/kg K
) = 1.1344 kJ/kg K
m₃ = 1 kg/s
so since:
m₁ + m₂ = m₃ (since m₃ = 1)
m₂ = 1 - m₁
From equation (2);
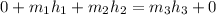
=
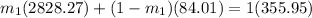
=
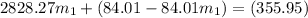
=
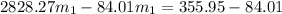
=
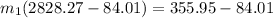
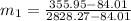
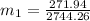
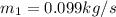
∴ the flow rate for steam from the boiler plant =
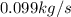
since; m₂ = 1 - m₁
m₂ = 1 - 0.099 kg/s
m₂ = 0.901 kg/s
∴ the flow rate from the city water = 0.901 kg/s
b)
rate of entropy production in the mixing tank can be determined using the formula:
Δ
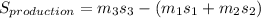
Δ
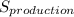
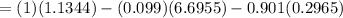
Δ
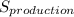
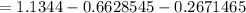
Δ

Δ
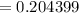
Δ
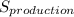 ≅ 0.2044 kJ/k
≅ 0.2044 kJ/k
∴ the rate of entropy production in the mixing tank = 0.2044 kJ/k