The question is incomplete, here is the complete question:
Consider the following multistep reaction:
C+D⇌CD (fast)
CD+D→CD₂ (slow)
CD₂+D→CD₃ (fast)
C+3D→CD₃
Based on this mechanism, determine the rate law for the overall reaction.
Answer: The rate law for the reaction is
![\text{Rate}=k'[C][D]^2](https://img.qammunity.org/2021/formulas/chemistry/college/8cl4jmixas09f0jw89ohdfkx6wny45cyxi.png)
Step-by-step explanation:
Rate law is the expression which is used to express the rate of the reaction in terms of the molar concentration of reactants where each term is raised to the power their stoichiometric coefficient respectively from a balanced chemical equation.
In a mechanism of the reaction, the slow step in the mechanism determines the rate of the reaction.
For the given chemical reaction:
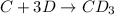
The intermediate reaction of the mechanism follows:
Step 1:
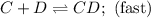
Step 2:
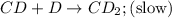
Step 3:
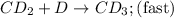
As, step 2 is the slow step. It is the rate determining step
Rate law for the reaction follows:
![\text{Rate}=k[CD][D]](https://img.qammunity.org/2021/formulas/chemistry/college/5xnfx1rgjoa0ashscyhonh01o7eelaruzu.png) ......(1)
......(1)
As, [CD] is not appearing as a reactant in the overall reaction. So, we apply steady state approximation in it.
Applying steady state approximation for CD from step 1, we get:
![K=([CD])/([C][D])](https://img.qammunity.org/2021/formulas/chemistry/college/jeswehkhx6jf6io24xjqwoeldlgyfkj0ug.png)
![[CD]=K[C][D]](https://img.qammunity.org/2021/formulas/chemistry/college/vhuyvkwqz4k43592lg2o1ckj14vo2xwx5e.png)
Putting the value of [CD] in equation 1, we get:
![\text{Rate}=k.K[C][D]^2\\\\\text{Rate}=k'[C][D]^2](https://img.qammunity.org/2021/formulas/chemistry/college/j57020i4qhkc7nj54ywwplmlslwzkbhbf8.png)
Hence, the rate law for the reaction is
![\text{Rate}=k'[C][D]^2](https://img.qammunity.org/2021/formulas/chemistry/college/8cl4jmixas09f0jw89ohdfkx6wny45cyxi.png)