Incomplete question as number of moles and length is missing.So I have assumed 3 moles and length of 0.300 m.So the complete question is here:
Three moles of an ideal gas are in a rigid cubical box with sides of length 0.300 m.What is the force that the gas exerts on each of the six sides of the box when the gas temperature is 20.0∘C?
Answer:
The Force act on each side is 2.43×10⁴N
Step-by-step explanation:
Given data
n=3 mol
L=0.3 m
Temperature=20.0°C=293 K
To find
Force F
Solution
To get force act on each side it would employ by
F=P.A
Where P is pressure
A is Area
First we need to find pressure by applying ideal gas law
So
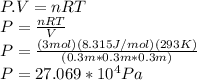
So The Force is given as:
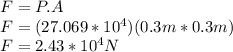
The Force act on each side is 2.43×10⁴N