Answer : The number-average molecular weight is, 97500 g/mol
Explanation :
First we have to calculate the molecular weight of isobutylene and isoprene.
The molecular formula of isobutylene and isoprene is,
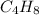 and
and
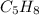
Molecular weight of
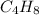 = 4(12 g//mol) + 8(1 g/mol) = 56 g/mol
= 4(12 g//mol) + 8(1 g/mol) = 56 g/mol
Molecular weight of
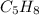 = 5(12 g//mol) + 8(1 g/mol) = 68 g/mol
= 5(12 g//mol) + 8(1 g/mol) = 68 g/mol
Now we have to calculate the average repeat molecular weight.
Average repeat molecular weight =
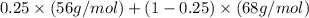
Average repeat molecular weight = 65 g/mol
Now we have to calculate the number-average molecular weight.
Number-average molecular weight = Average repeat molecular weight × Degree of polymerization
Number-average molecular weight = (65 g/mol) × (1500)
Number-average molecular weight = 97500 g/mol
Thus, the number-average molecular weight is, 97500 g/mol