Answer The value of
 and
and
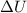 is, 40.79 kJ and 37.7 kJ respectively.
is, 40.79 kJ and 37.7 kJ respectively.
Explanation :
Heat released at constant pressure is known as enthalpy.
The formula used for change in enthalpy of the gas is:
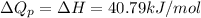
Now we have to calculate the work done.
Formula used :
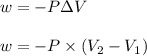
where,
w = work done = ?
P = external pressure of the gas = 1 atm
 = initial volume =
= initial volume =
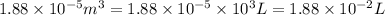
 = final volume =
= final volume =
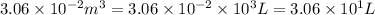
Now put all the given values in the above formula, we get:
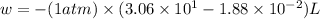
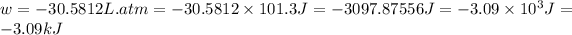
Now we have to calculate the change in internal energy.
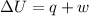
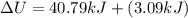
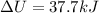
Thus, the value of
 and
and
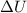 is, 40.79 kJ and 37.7 kJ respectively.
is, 40.79 kJ and 37.7 kJ respectively.