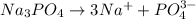Answer: Sodium sulfate and Ammonium sulfate could not be used for this purpose.
Step-by-step explanation:
An acidic solution will show a pH in the range of 0 - 6.9. A basic solution show a pH in the range of 7.1 - 14. A neutral solution will show a pH of 7.
A salt is acidic, when it is formed from the combination of strong acid and weak base. A salt is basic, when it is formed from the combination of weak acid and strong base. A salt is neutral, when it is formed from the combination of weak acid and weak base or strong acid and strong base.
a. Sodium carbonate : is formed by the combination of strong base
 and a weak acid
and a weak acid
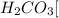 , thus forms a basic solution.
, thus forms a basic solution.
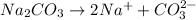
b. Sodium sulfate: is formed by the combination of strong base
 and a strong acid
and a strong acid
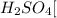 , thus forms a neutral solution.
, thus forms a neutral solution.
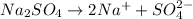
c. Ammonium sulfate: is formed by the combination of weak base
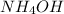 and a strong acid
and a strong acid
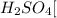 , thus forms a acidic solution.
, thus forms a acidic solution.
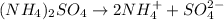
d. Sodium phosphate: is formed by the combination of strong base
 and a weak acid
and a weak acid
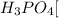 , thus forms a basic solution.
, thus forms a basic solution.