Step-by-step explanation:
It is given that possible number of ways the Cl and Br can be absorbed initially are 100.
S, possible number of ways by which Br can be desorbed is as follows.
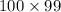
Now, we will calculate the change in entropy as follows.
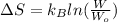
where,
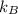 = Boltzmann constant =
= Boltzmann constant =
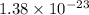
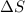 = change in entropy
= change in entropy
Therefore, we will calculate the change in entropy as follows.
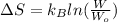
=
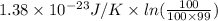
=
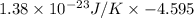
=
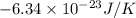
Thus, we can conclude that the change in entropy is
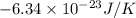 .
.