Step-by-step explanation:
Expression for the
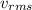 speed is as follows.
speed is as follows.
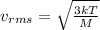
where,
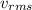 = root mean square speed
= root mean square speed
k = Boltzmann constant
T = temperature
M = molecular mass
As the molecular weight of oxygen is 0.031 kg/mol and R = 8.314 J/mol K. Hence, we will calculate the value of
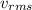 as follows.
as follows.
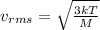
=
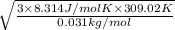
= 498.5 m/s
Hence,
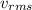 for oxygen atom is 498.5 m/s.
for oxygen atom is 498.5 m/s.
For nitrogen atom, the molecular weight is 0.028 kg/mol. Hence, we will calculate its
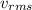 speed as follows.
speed as follows.
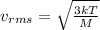
=
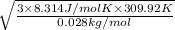
= 524.5 m/s
Therefore,
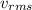 speed for nitrogen is 524.5 m/s.
speed for nitrogen is 524.5 m/s.