Answer:
It will take 313.376 sec to raise temperature to boiling point
Step-by-step explanation:
We have given that potential difference V = 120 Volt
Current i = 4.50 A
So resistance
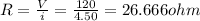
Heat flow in resistor will be equal to
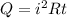
It is given that this heat is used for boiling the water
Mass of the water = 0.525 kg = 525 gram
Specific heat of water 4.186 J/gram/°C
Initial temperature is given as 23°C
Boiling temperature of water = 100°C
So change in temperature = 100-23 = 77°C
Heat required to raise the temperature of water
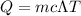
So
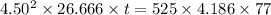
t = 313.376 sec
So it will take 313.376 sec to raise temperature to boiling point