Answer:
In 23.49 minutes the concentration of A to be 66.8% of the initial concentration.
Step-by-step explanation:
The equation used to calculate the constant for first order kinetics:
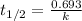 .....(1)
.....(1)
Rate law expression for first order kinetics is given by the equation:
![t=(2.303)/(k)\log([A_o])/([A])](https://img.qammunity.org/2021/formulas/chemistry/college/kjsezcfx0d6b1205bo3kaekpswf8f4fztx.png) ......(2)
......(2)
where,
k = rate constant
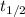 =Half life of the reaction =
=Half life of the reaction =

t = time taken for decay process = ?
![[A_o]](https://img.qammunity.org/2021/formulas/physics/college/3jrctnxyrdjmiz9ngr0s6o9r3hdvpo6qhe.png) = initial amount of the reactant = 0.163 M
= initial amount of the reactant = 0.163 M
[A] = amount left after time t = 66.8% of
![[A_o]](https://img.qammunity.org/2021/formulas/physics/college/3jrctnxyrdjmiz9ngr0s6o9r3hdvpo6qhe.png)
[A]=
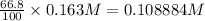
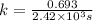
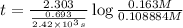
t = 1,409.19 s
1 minute = 60 sec
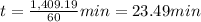
In 23.49 minutes the concentration of A to be 66.8% of the initial concentration.