Answer:
D.V.= - 1.34 %
Step-by-step explanation:
Given that
V₁=50,000 L
T₁=25⁰C = 273 + 25 K =298 K
The final temperature ,T₂= 25 - 4 ⁰C = 21 ⁰C
T₂ = 21 + 273 K= 294 K
We know that for constant pressure process
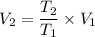
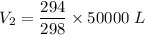
V₂=49328.85 L
The percent decrease in the volume is given as
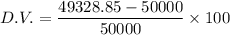
D.V.= - 1.34 %