Answer:
The correct answer is:
5×5700 yr = 28,500 years (d)
Step-by-step explanation:
The half life of an element/atom is the time is takes for that element/atom to decay to half of its original mass/size.
It was found that the carbon-14 atom had decayed until only
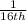 of the original size is still remaining.
of the original size is still remaining.
Now, let us first find how many half lives are in one-sixteenth, and that is
 =
=
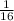 . so it means that there are 4 half lives in one-sixteenth, and since one half life takes approximately 5700 years, therefore 4 half lives will take 4×5700 yr = 22, 800 years, but at this point (22,800 years ago)the fossil has already half-life, therefore the additional age is to add the number of years it took to decay that half life. hence the age of fossil is:
. so it means that there are 4 half lives in one-sixteenth, and since one half life takes approximately 5700 years, therefore 4 half lives will take 4×5700 yr = 22, 800 years, but at this point (22,800 years ago)the fossil has already half-life, therefore the additional age is to add the number of years it took to decay that half life. hence the age of fossil is:
22,800 + 5700 = 28, 500 years