Answer:
volume of gas = 9.1436cm³
Step-by-step explanation:
We will only temperature from °C to K since the conversion is done by the addition of 273 to the Celsius value.
Its not necessary to convert pressure and volume as their conversions are done by multiplication and upon division using the combined gas equation, the factors used in their conversions will cancel out.
V1 =10.1cm³ , P1 =746mmHg, T1=23°C =23+273=296k
V2 =? , P2 =760mmmHg , T2=0°C = 0+273 =273K
Using the combined gas equation to calculate for V2;
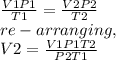
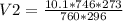
V2=9.1436cm³