Ammonia is prepared industrially by the reaction:
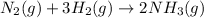
for the reaction: ΔH° = –92.2 kJ and K (at 25°C) = 4.0 × 10⁸. When the temperature of the reaction is increased to 500°C, which of the following is true?
a. At equilibrium, more NH₃ is present at 500°C than at 25°C.
b. The reaction of N₂ with H₂ to form ammonia is endothermic.
c. K for the reaction will be larger at 500°C than at 25°C.
d. Product formation (at equilibrium) is not favored as the temperature is raised.
e. None of these choices is true.