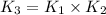Answer : The equilibrium constant expression for overall reaction will be:
Step-by-step explanation:
The given chemical reactions are:
Step 1 :
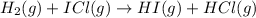 ;
;
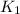
Step 2 :
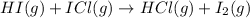 ;
;
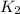
Overall :
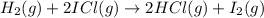 ;
;
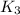
By the addition of reaction 1 and reaction 2, we get the overall reaction.
As we know that, when we are adding reactions then their equilibrium constant will be multiplied.
Thus, the equilibrium constant expression for overall reaction will be: