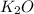Answer : The empirical formula for the given compound is
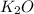
Explanation :
Step 1: Converting the given masses into moles.
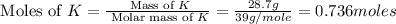
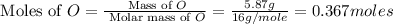
Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 0.367 moles.
For Potassium =
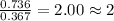
For Oxygen =
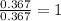
Step 3: Taking the mole ratio as their subscripts.
The ratio of K : O = 2 : 1
Hence, the empirical formula for the given compound is