Answer:
C - 12.01 g/mol
H - 1.008 g/mol
CH - 13.018g/mol
molecular formula = n×(empirical formula)
78.11 g/mol = n ×( 12.01 + 1.008) g/mol
∴ n = 5.99
≈ 6
then
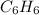
Explanation: First find the molar mass of the empirical formula CH.
use the formula that says: Molecular formula = n × empirical formula
therefore you divide the molar mass of the compound by molar mass of the empirical formula.
the empirical is the 6th and then multiply that 6 with the subscripts of CH.