Answer:
The correct answers are: Dilution factor = 250 and concentration of DNA in undiluted sample = 2.5 μg/μl.
Step-by-step explanation:
- Volume of DNA taken = 2 μl.
- Volume of added water = 498 μl.
- Total volume of the sample = 2 μl + 498 μl = 500 μl.
- Dilution factor =
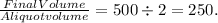
- A₂₆₀ of the given sample is = 0.2.
- We know that 50 μg/ml concentration of pure double stranded DNA gives an absorbance, A₂₆₀ = 1.
- Again, Concentration of DNA(µg/ml) =
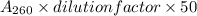 µg/ml.
µg/ml. - =
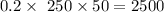 μg/ml.
μg/ml. - In order to convert it into μg/μl, we have to divide the above value by 1000, hence we get,
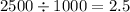 μg/μl.
μg/μl.