Answer:
dealing with a buffer
use the formula C = n/V...........1
therefore say n = m/Mr.........2
then substitute equation 2 into equation 1
∴ CV = m/Mr
then m = CVMr
Since we are looking for mass of Nacl to make a buffer solution.
m = 150×
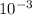 m
m
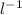 × 500×
× 500×
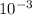 l × 40 g/mol
l × 40 g/mol
mass of Nacl = 3g
Explanation: You first weight out 3g of 150mM of NaCl and then dissolve it into a 1000ml beaker dilute it using a volume of 500ml of water to make volume.