Answer:
- The student should use 250g of the 14.0% w/w solution of thiophene in ethanol
Step-by-step explanation:
You must find how many grams of a 14.0% solution contains 35.0 g of thiophene (solute) and then evaluate if the amount available (1.0 kg) is enough.
Formula:
- % w/w = (mass of solute / mass of solution) × 100
Substitute and solve for the mass of solution:
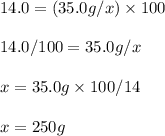
Hence, the student should use 250g of the 14.0% w/w solution of thiophene in ethanol. Since, 1.0 kg is 1,000g there is enough available.