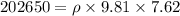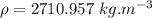Answer:
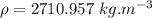
Step-by-step explanation:
Given:
height of the given liquid in the tank,
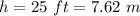
pressure at the surface of the liquid,
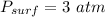
pressure at the bottom of the liquid,
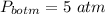
So the pressure due to height of the liquid column:
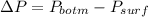
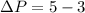
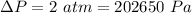
Now as we know that the pressure due to the height of liquid column is given as:
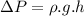
where:
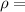 density of the liquid
density of the liquid
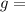 acceleration due to gravity
acceleration due to gravity