Answer: 7.79 grams of ethanol were put into the beaker.
Step-by-step explanation:
To calculate the mass of ethanol, we use the equation:
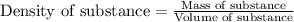
Density of ethanol = 0.779 g/mL
Volume of water = 10.00 mL
Putting values in above equation, we get:
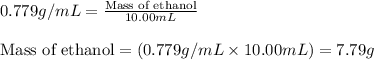
Thus 7.79 grams of ethanol were put into the beaker.