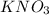 (potassium nitrate) is least soluble in water at 20°c.
(potassium nitrate) is least soluble in water at 20°c.
Step-by-step explanation:
The deciding element for the outcome is the dissolvability of the substance, which is characterized as the greatest conceivable centralization of the solute. The solvency rules help figure out which substances are solvent, and to what degree. Dissolvability demonstrates the greatest measure of a substance that can be broken down in a dissolvable at a given temperature. Such an answer is called soaked. Separation of the mass of the compound by the mass of the dissolvable and afterward duplicate by 100 g to figure.