0.3725 grams
Step-by-step explanation:
Step-1
The chemical formula for ammonium phosphate is (NH4)3PO4.
Each molecule of ammonium phosphate has 3 nitrogen atoms, 12 hydrogen atoms, one phosphorous atom and 3 oxygen atoms.
Step-2
Let us calculate molecular weight of ammonium phosphate
Atomic weight of Nitrogen = 14
Atomic weight of Hydrogen = 1
Atomic weight of Phosphorous = 31
Atomic weight of Oxygen = 16
Molecular weight of Ammonium phosphate = 3*14+12*1+1*31+4*16=149
One mole of ammonium phosphate weighs 149 grams.
Hence 2.5*
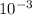 moles will weigh 2.5 *
moles will weigh 2.5 *
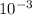 * 149 = 372.5 *
* 149 = 372.5 *
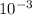 =0.3725 grams.
=0.3725 grams.