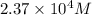Answer : The concentration of
 needed is,
needed is,
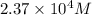
Explanation :
First we have to calculate the mole of phosphate.
As we are given that, 1 mg P/L that means, 1 mg of phosphate present in 1 L of solution.
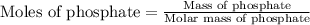
Molar mass of phosphate = 94.97 g/mole
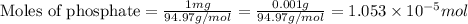
Now we have to calculate the concentration of phosphate.
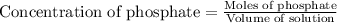
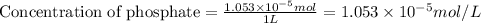
Now we have to calculate the concentration of
 .
.
The second equilibrium reaction is,
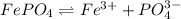
The solubility constant expression for this reaction is:
![K_(sp)=[Fe^(3+)][PO_4^(3-)]](https://img.qammunity.org/2021/formulas/chemistry/college/4qnwpcr2m1j0zg1kswq08yca05nhu255g5.png)
Given:
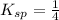
![(1)/(4)=[Fe^(3+)]* 1.053* 10^(-5)mol/L](https://img.qammunity.org/2021/formulas/chemistry/college/qs58mbch1muy47icu5p3y52y5jqcnwej51.png)
![[Fe^(3+)]=2.37* 10^4M](https://img.qammunity.org/2021/formulas/chemistry/college/suotbb5gmccnstzafyxe2a6chy69564nec.png)
Thus, the concentration of
 needed is,
needed is,